The terminal voltage of a battery is less than its EMF because of the internal resistance inside the battery that causes a voltage drop when current flows through it. In simple terms, EMF (electromotive force) is the total energy supplied by the chemical reaction within the battery, while the terminal voltage is the actual voltage available across the terminals when the battery is delivering current to a load.
As current flows, some energy is lost inside the battery due to its internal resistance, reducing the voltage that reaches the external circuit. This is why the terminal voltage is always slightly less than the EMF during the discharging process.
Let us understand in detail how it happens.
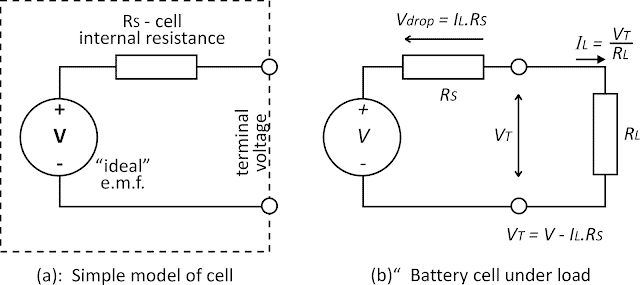
Equivalent Circuit of Battery
The equivalent circuit of the battery is given below. A voltage source can be represented as an EMF source with internal series resistance.
Battery Cell Under No-Load Condition
Under no load condition, the current flowing through the battery or cell is zero, and the terminal voltage is exactly equal to the EMF of the cell or battery.
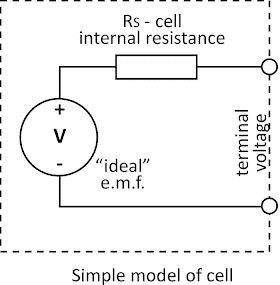
Battery Cell Under Load Condition
When the cell or battery is connected to the load, the battery supplies current to the load. Now, if we measure the voltage across the load ( that is the same point at battery terminals), the measured voltage will be somewhat less than the battery EMF.
The measured voltage at the terminal of the battery when the battery supplies current to load is called the terminal voltage.
Reason for Reduction in Battery Voltage
What is the reason for the reduction in the battery voltage? There must be some voltage drop inside the battery. Yes, the voltage drop takes place inside the cell because of the internal resistance of the battery. The internal resistance of the voltage source is zero for an ideal voltage source.
However, the voltage source can never be an ideal source and its internal resistance can not be zero. The internal resistance of the battery or cell can be made as minimum as possible by selecting the proper metallurgy for the battery materials.
Effect of Internal Resistance on Terminal Voltage
The voltage source has some internal resistance which causes the voltage to drop during discharging of the battery, and the terminal voltage will be less than the EMF of the battery at the time of discharge. Does the terminal voltage is same for all types of loads? No, it depends upon the value of the load. The higher the value of the load, the more will be current from the battery and consequently, the terminal voltage will be less.
Explanation Using Kirchhoff’s Voltage Law
Let us understand this phenomenon with Kirchhoff’s voltage law.
In the above circuit, the load is infinitive and the current flowing through the battery is zero. The voltage drop across the internal resistance is ILRs is equal to zero because IL is zero, and in this condition, the battery is said to be open-circuited. In open-circuited conditions, the battery terminal voltage is equal to the EMF of the battery.
Battery Under Load Condition
The cell voltage having EMF V is connected to the load RL. The cell supplies current IL to the load. According to Kirchhoff’s voltage law sum of the potential drops in a closed-loop electric circuit is zero.
V = ILRs + ILRL
V = ILRs + (VT/RL) RL
V = ILRs + VT
If the battery is open circuit, IL=0
V =ILRs + ILRL
V=0 x Rs + (VT/RL) RL
V=0 + VT
VT =V
When the battery is not connected to the load the load current is zero and the battery EMF is equal to the terminal voltage.
Battery Connected to Load
Now if the load is connected to the battery, the battery starts supplying the current to the load.
V = ILRs + ILRL
V = ILRs + (VT/RL) RL
V = ILRs + VT
VT = V- ILRs
From the above, it is clear that the terminal VT voltage is less than the EMF of the battery. The reason for less terminal voltage when the battery is under load is voltage drop inside the battery caused by battery internal resistance Rs.
Effect of Load Resistance on Terminal Voltage
The terminal voltage further depends on the magnitude of the load. The load current depends on the value of the load resistance. The lower value of load resistance causes to draw more current from the battery, and consequently, more voltage drop takes place inside the battery, and there will be less terminal voltage. Let us understand this with an illustrative example.
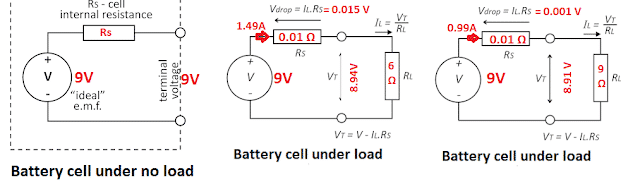
Illustrative Examples of Battery Terminal Voltage
Case 1: When a battery is under no load
When there is no current flowing through the battery, the terminal voltage is equal to the battery EMF. The battery EMF is 9 volts and the terminal voltage is equal to the battery EMF which is 9 volts.
Case 2: When a battery is under load – Load is 9 Ω
When the battery of 9 volts EMF is connected to 9 Ω resistance the battery will supply 1.49 amp. current to the circuit and the terminal voltage is 8.94 volts. The voltage drop across the internal resistance of the battery is 0.015 volts.
Case 3: When a battery is under load – Load is 6 Ω
When the battery of 9 volts EMF is connected to 6 Ω resistance the battery will supply 0.99 amp. current to the circuit and the terminal voltage is 8.91 volts. The voltage drop across the internal resistance of the battery is 0.001 volts.
From above, it is clear that voltage drop across the internal resistance increases with an increase in the current.
When there is no current flowing through the battery, the terminal voltage is equal to the battery EMF. The battery EMF is 9 volts and the terminal voltage is equal to the battery EMF which is 9 volts.
Relationship Between Terminal Voltage and Load Current
The terminal voltage decrease with an increase in load current or the current through the battery. The graph showing relationship between voltage and current is shown below.
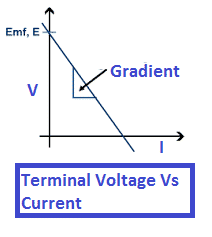
The relationship between terminal voltage and load current of a battery can be represented by a straight-line graph.
When no current flows (open circuit), the terminal voltage is equal to the EMF of the battery. As the current increases, a voltage drop occurs across the internal resistance of the cell, causing the terminal voltage to decrease linearly.
If we plot the terminal voltage V on the y-axis and the load current I on the x-axis, the graph appears as a straight line with a negative slope. The slope of the line represents the internal resistance r of the battery.
At:
- Zero current (I = 0) → V=E (the intercept of the graph)
- Increasing current → Vgradually decreases
- Very high current → V becomes significantly less than E
This linear relationship can be expressed mathematically as:
V=E−Ir
Thus, the graph clearly shows that the terminal voltage falls as the load current increases due to the internal resistance of the battery.
Solved Problem on Battery Terminal Voltage
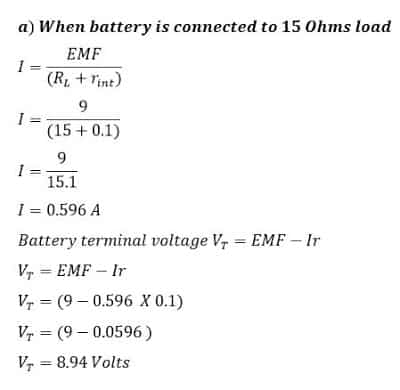
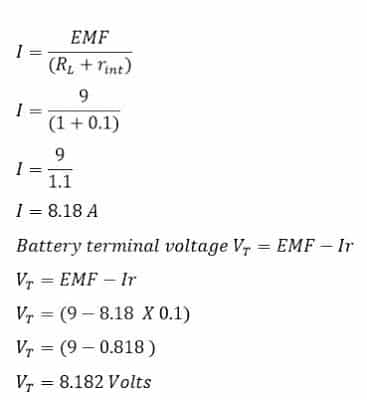
A battery has 9 volts emf and internal resistance of 0.1 Ohms. Calculate
a) Calculate battery terminal voltage when it is connected to 15 Ohms load.
b) The terminal voltage when it is connected to a 1.0 Ohms load.
b) When the battery is connected to 1.0 Ohms load
Key Takeaways
- Terminal voltage = EMF under no-load condition.
- During discharge, internal resistance causes voltage drop.
- Heavier load → more current → more voltage drop → lower terminal voltage.
Conclusion
The terminal voltage of a battery is always less than its EMF during discharge because of the voltage drop across the internal resistance of the battery. When the battery delivers current to an external load, part of the energy is lost inside the cell, resulting in a lower voltage at the terminals. Under no-load conditions, when no current flows, the terminal voltage becomes equal to the EMF.
The greater the load current, the larger the internal voltage drop, and the smaller the terminal voltage. Hence, maintaining low internal resistance through good battery design and materials is essential for achieving higher efficiency and stable performance.

Related Articles: